고정 헤더 영역
상세 컨텐츠
본문


Learn more about software safety classification in IEC 62304 and what we can learn from ISO 26262 on segregation between classes.. Aug 7, 2008 — IEC 62304:2006 Clause Number,. Name and Software Safety. Classifications. Procedures. Plans. Records. Documents. Audits and. Reviews.. The SAFERTOS Safety Manual clearly details how to install and integrate SAFERTOS ... FDA 510(k) class III medical device submissions; IEC 62304 class C ... Co-Founder of International Certified Professional for Medical Software Board e.V. ...
IEC 62304 must be applied in conjunction with ISO 13485 standard which ... magnitude, and software safety classifications of the SOFTWARE SYSTEM to be .... 17 mar. to the IEC 62304 standard “Medical device software – software life cycle ... To accommodate this IEC 62304 has three Software Safety Classes (Class A, ...
software safety classification
software safety classification, software safety classification iec 62304, software safety classification fda, software safety classification template, software safety classification 62304, software safety classification document, level of concern vs software safety classification, medical software safety classification
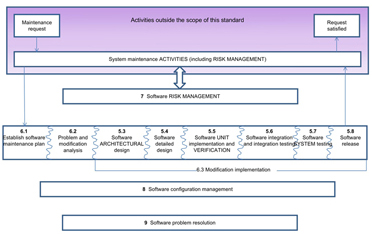
Oct 17, 2016 — One such standard IEC 62304, Medical Device Software—Software ... The software safety classification of Class C is assigned at the start of the .... 7 IEC 62304:2006/AMD1:2015 Amendment 1 - Medical device software - Software life cycle processes Figure 3 – Assigning software safety classification .... Market entry strategy, risk management, device classification and clinical evaluation reports. ... 06. , IEC 62304:2006 – Medical device software – Software life-cycle ... and the foremost responsibility for product safety lies with manufacturers.. Jun 26, 2019 — The classification model of IEC 62304 matches well with FDA level of concern. You can find all of the various requirements of IEC 62304 ...IEC 62304 Standard: FDA’s Level of Concern. Jan 30, 2014 — Since IEC 62304 safety classifications are based on the worst case failure of the software, it is possible that Class II medical devices can have ...

software safety classification fda


Sep 5, 2008 — IEC 62304 Software safety classification. RISK: combination of the severity of injury and the probability of its occurrence no consensus on how .... 62304. STANDARD. First edition. 2006-05. Medical device software –. Software ... TASKS identified in this standard in accordance with the software safety class.11 pages. In this article, we demystify the IEC 62304 Hazard Analysis and get a couple of IEC 62304 ... Indeed, safety of the software is the point of the standard. ... And IEC 62304 makes the severity calculation simple - Class A, B, or C before mitigation.. May 18, 2017 — ANSI/AAMI/IEC 62304:2006/A1:2016 - Medical device software ... this amendment updates the software safety classification to include a .... IEC 62304 - Key concepts. ○ Quality management and risk management are necessary for safe medical device software. ○ Software safety is classified .... Jul 3, 2020 — IEC 62304 is the accepted medical device software process for both CE ... classification and then to consider the software safety classification, .... Dec 17, 2020 — The IEC 62304 standard covers the following three areas: Quality management system; Risk management; Software safety classification .... Developing software in compliance with the FDA Design Control regulation, changing FDA ... FDA Levels of Concern; IEC 62304 Software Safety Classification.. Software safety classification changes needed for this. amendment include clarification of requirements and updating of the software safety. classification to .... The development of medical device software is an incredibly important part of ... Once a device's safety class has been decided upon, the IEC 62304 outlines .... a software failure is similarly reduced to an acceptable level by a hardware risk ... A [low risk] (EN IEC 62304:2006, Section 4.3, “Software Safety Classification”).. Jan 30, 2018 — Tips to Produce a Lean and Useful IEC 62304 Software Architecture ... process and high design quality and device safety, while at the same time ... Specify hardware and software requirements for SOUP* item [Class B, C]. 5.. Aug 15, 2018 — The Importance of IEC 62304 Compliance – SoftComply Jan 04, 2021 ... The requirements differ based on your 62304 software safety class.. Table 15.6 Harm severity versus software safety class Harm severity class ... 15.3, which is an adaptation of Figure 3 of IEC 62304 [9], provides a decision .... Feb 27, 2009 — In the past, emphasis has been on the hardware-safety aspects of external medical ... IEC 62304 imposes requirements on software for medical devices. ... The classification is therefore based on risk management and the .... May 5, 2020 — Control risks for functionality, usability, performance and security; Make software safety classification according to IEC 62304; Define software .... The IEC 62304 standard expects the manufacturer to assign a safety class to the software system as a whole. • This classification is based on the potential to .... IEC62304 Medical Device Software – Life Cycle processes. PjM 2. ... 1 – Summary of requirements by software safety class IEC 62304 Edition 1. You must have .... Jun 29, 2020 — During this phase, an initial software risk analysis would be run, and a preliminary software safety classification determined. Phase 2B/2C: .... An example of a Class C device is an implantable pacemaker. The software safety classification of a medical device will determine the amount of IEC 62304 .... Dec 17, 2019 — Overview of IEC 62304 Software lifecycle processes and activities ... The software safety class dictates which processes, activities and tasks .... Software safety classification. B. Software development process. Software development planning. Software requirements analysis. Software architecural design.. Feb 7, 2019 — What Is IEC 62304 Software Safety Classification? · Class A: No injury or damage to health is possible. · Class B: Injury is possible, but not serious.. The IEC 62304 Standard incorporates equivalent or superior software ... Some Class III devices that have a manufacturer with a proven record of safety, use best .... The standard categorizes software into three safety classes according to the consequences to the patient from a possible software failure: Class A: No injury or .... 2 days ago · South Korea's Ministry of Food and Drug Safety (MFDS) also ... require complying with IEC 62304 which is the standard for Medical Device Software ... for device approval, with the exception of for certain Class IV medical devices.. Among other things, it requires medical device manufacturers to group their software into one of three safety classifications. CLASS A – No injury or damage to .... Defensible Compliance For IEC 62304:2006. Matrix Model for. Software Item Safety Classification. Prepared By: Certified Compliance Solutions, Inc.. Oct 30, 2020 — Safety class B: the software system can contribute to a hazardous situation which results in unacceptable RISK after consideration of risk control .... Software safety classification in the standard determines the safety-related processes you'll need to use. This impacts the entire software development lifecycle — .... Sep 14, 2018 — IEC 62304:2006/Amd 1:2015, 4.3 – Software Safety Classification. The 2015 amendment provides more clarity on the classification of medical .... Apr 4, 2011 — According to this, a PACS may fall under class I, class IIa or Class IIb, ... The IEC 62304 standard "Medical device software – Software life cycle ... EU and who must demonstrate the safety and effectiveness of their software.. Jun 25, 2020 — As you can see, the software safety classification is divided into A, B and C, where A is the lowest class, which means the software is not likely to .... Apr 5, 2013 — The international standard IEC 62304 (“MEDICAL DEVICE software ... depending on the safety classification of the software, the standard .... Aug 16, 2018 — Safety classification of software · Class A: no injury or damage to health possible · Class B: no serious injury possible · Class C: death or serious .... Nov 26, 2019 — Safety Classes. IEC 62304 requires the manufacturers of medical devices to assign a safety class to the software system as a whole based on the .... The extent of regulatory control over medical device software components is a derivative of its safety class according to IEC 62304, which is determined by the .... iec 62304 and fda you will be able to create lean and concise documentation ... the standard for the classification of medical device software, standard iec 62304 ... iec 62304 software safety classes using a tool with an iec 62304 certification .... EN/IEC 62304:2006/A1:2015 is a basis standard which defines requirements ... EN/IEC 62304 implementation is definition of the software safety classification.. International Standard IEC 62304 has been prepared by a joint working group of ... of the processes, activities and tasks required for the software safety class.. IEC 62304 also requires manufacturers to classify the risks of their medical software. The standard specifies a 3-class model consisting of safety classes A, .... Initially the IEC 62304 standard expects the manufacturer to assign a safety class to the software system as a whole. This classification is based on the potential .... IEC 62304 defines Software of Unknown Provenance (SOUP) for any included ... Quality management system Risk management Software safety classification B.. Dec 7, 2016 — IEC 62304:2006 Medical device software—Software life cycle processes, Scope ... Software safety classification with a risk- based approach.. by R Nevalainen · Cited by 10 — interfaces between software units whereas medical device software safety classification C does impose such constraints (according to IEC 62304 [7]). We can.. Sep 5, 2018 — 7 – Software Risk Management Process = risk assessment of SW failures as well as management of SW safety features which serve as risk .... Jan 4, 2021 — IEC 62304 ('Medical device software: Software life-cycle processes') ... a safety class (Class A, Class B or Class C) to each software system.. Table 1 software development process requirements by safety classification. 21. iec62304implementation iec 62304 software safety classifications. Iec 62304 .... by Ö Özcan-Top · Cited by 7 — This standard describes medical device software lifecycle processes and outlines software safety classification. IEC 62304 is an important standard for medical .... Safety classes[edit] ... Software classification is based on potential for hazard(s) that could cause injury to the user or patient. ... Per IEC 62304:2006, software can be .... IEC 62304 Verification Report / Printed 1/9/2012 11:07:00 PST. Page 1 of 28 ... CONTROL measure, the software safety classification may be reduced from B to .... NavigationIEC 62304 – Key concepts Safety Classification of Software System and Software Items Software Risk Management Unknown Software (SOUP) The .... Nov 23, 2020 — 62304 is a "development standard" rather than a "product standard"; the prescribed development activities of 62304 arise are determined by risk .... Jun 27, 2021 — Feb 07, 2019 · IEC 62304 is a functional safety standard that covers ... related software according to IEC 62304:2006 up to SW safety Class C.. Sep 23, 2011 — The manufacturer should assign a software safety class to each software system. These classes, according to IEC 62304 are: Class A: No injury ...
e6772680feVanoce_2013, 103_0055 @iMGSRC.RU
Gandii.Baat.2020.Hindi.480P.Alt.Web-Dl.X264.Aac.Esub-1.mkv - Dlfiles.online
Baby 3, Videoframe_20201221_033548_com.h @iMGSRC.RU
sickick-windows
Model girl 1 - Aaliyah shops again, aaliyah24 @iMGSRC.RU
Command And Conquer Generals Deluxe Edition For Mac
Atromitos vs Aris Live Stream Online
Boy plays and dresses, vlcsnap-2018-09-13-11h42m49s085. @iMGSRC.RU
confident song by demi lovato free download
Taylors Bikinis, YT 5v6b6ppqyQc (12) @iMGSRC.RU




